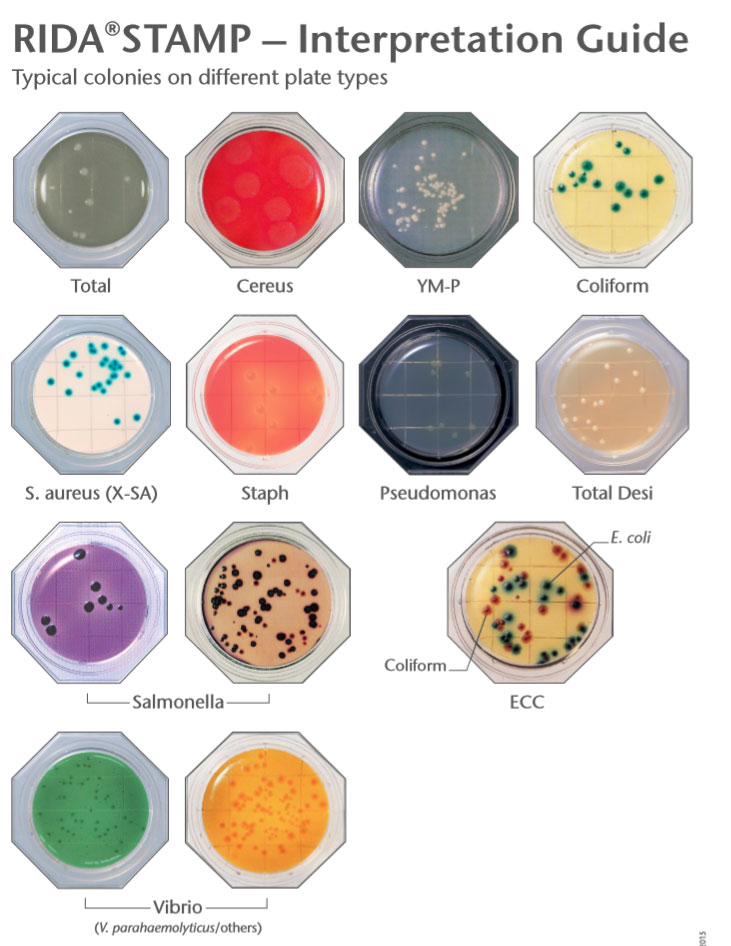
How to Improve Your Factory Hygiene
The main goal of any food manufacturer is to ship a product to your customer that is safe to eat and this means that it should be microbiologically stable till consumed. In order to do this your food must be produced in an environment that is tightly controlled and this includes adequate plant hygiene.
The first criteria for adequate hygiene is to have your factory hygienically designed according to good manufacturing practice. It is important to use the correct materials and design for floors, walls and ceiling in order to have the highest possible hygiene standards. The following link gives a detailed guideline on how to design a hygienic factory. Besides a good factory layout and construction, the equipment also have to be made according to good manufacturing practice. The following video link provides an insight into sanitary equipment design.
How to Improve Ready To Eat Food Processing Plants Through Sanitary Design
Factory Cleaning and Disinfection
Factories must be cleaned and disinfected after every production cycle and procedures to ensure that this is done in an effective and timely manner and must be documented. This will include decisions on when to clean, what chemicals to use, the strengths of the disinfectants used. These procedures are the heart of a good GMP practice. The final and most important part of these cleaning procedures is to have a mechanism to check that the cleaning has been correctly carried out. This process is called cleaning validation. Cleaning is a process of removal of residues that are reminiscent of the food production process. In order to make sure that the cleaning in done properly it is necessary to monitor the effectiveness of the cleaning procedure. The cleaning cycle is in a constant state of flux due to changing personnel and changes in process.
ATP as A Proxy for Cleaning Efficiency
Multidrug-resistant microorganisms and the current coronavirus (COVID-19) pandemic have raised public awareness of the need to control the spread of infectious diseases. It is becoming ever more important that public services monitor the effectiveness of cleaning and disinfection procedures. Recent studies by the NIH (National Institutes of Health) showed that viable viruses can be detected on surfaces after 2-3 days.
Operational hygiene teams are responsible for verifying that cleaning procedures are properly implemented, followed, and continue to be proven effective. MVP ICON® is a quick and easy-to-use system to verify the effectiveness of cleaning and disinfection procedures on a routine basis. The system is intended to measure the amount of adenosine triphosphate (ATP) on cleaned surfaces, thereby providing a quantifiable indication of cleaning effectiveness, even if viruses like SARS-CoV-2 are not directly detected.
Reference: Sifuentes L.Y., Fankem, S.L.M., Reynolds, K., Tamimi, A.K., Gerba, C.P., Koenig, D. (2017): Use of ATP Readings to Predict a Successful Hygiene Intervention in the Workplace to Reduce the Spread of Viruses on Fomites. Food Environ Virol 9:14–19
Methods of measurement

Traditional microbiology
- Measures only viable organism
- Result in 2-7 days
- Not effective in real time monitoring
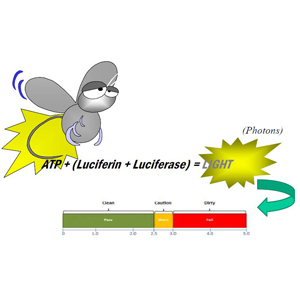
ATP bioluminescence
- Measures total biological residue
- Result immediate actionable results
- Better indication of cleaning effectiveness
- Without food residue no bacterial growth

The MPV ICON – one instrument does it all.
Designed to monitor AND manage your HACCP and Hygiene monitoring program
Besides hygiene monitoring, the icon can be used to check that the sanitizing agent and chlorine bath is prepared to its correct strength.
The non-glass temperature and pH probe enables you to check process temperatures and pH.

The MPV ICON – one instrument does it all.
Besides hygiene monitoring, the icon can be used to check that the sanitizing agent and chlorine bath is prepared to its correct strength.
The non-glass temperature and pH probe enables you to check process temperatures and pH.
Personnel Hygiene
Personnel hygiene is one of the most important factor in food safety as most contamination comes from transfer of microbes from people to the food surfaces. Proper design and specification of clothing and foot wear and management of workflow and hygiene control at various zones help curb the transfer of bacteria.
Microbial Air Sampling
Air borne microbes is a significant source of contamination for products. Contamination is a dangerous enemy and its source often comes from air conditioning and the flow of people and goods into the production floor. Therefore air sampling is used to gauge the quality of air in terms of particulates, fungi, spores, and microorganisms – even of viruses and bacteriophages.
Water Quality Monitoring
Water is often a direct ingredient in food manufacturing. The drinking water quality in Singapore is regulated by the Environmental Public Health Regulations 2019.
Food factories normally check the water supply for accidental contamination of the water supply by checking for E. coli and coliform. For microbiological examination of water, the Singapore standards require a sample of 100ml to be free of E. coli and coliform. The most convenient way to carry this out is to use membrane filtration as in the video on the left.

We provide the best services about science.
About Company
Sitemap
Please contact our friendly sales staff for more information.
Feel free to ask us questions. We would love to assist you !